Microbiological Environmental Monitoring
for pharmacies, pharma and medicine
Our Satisfied Customers



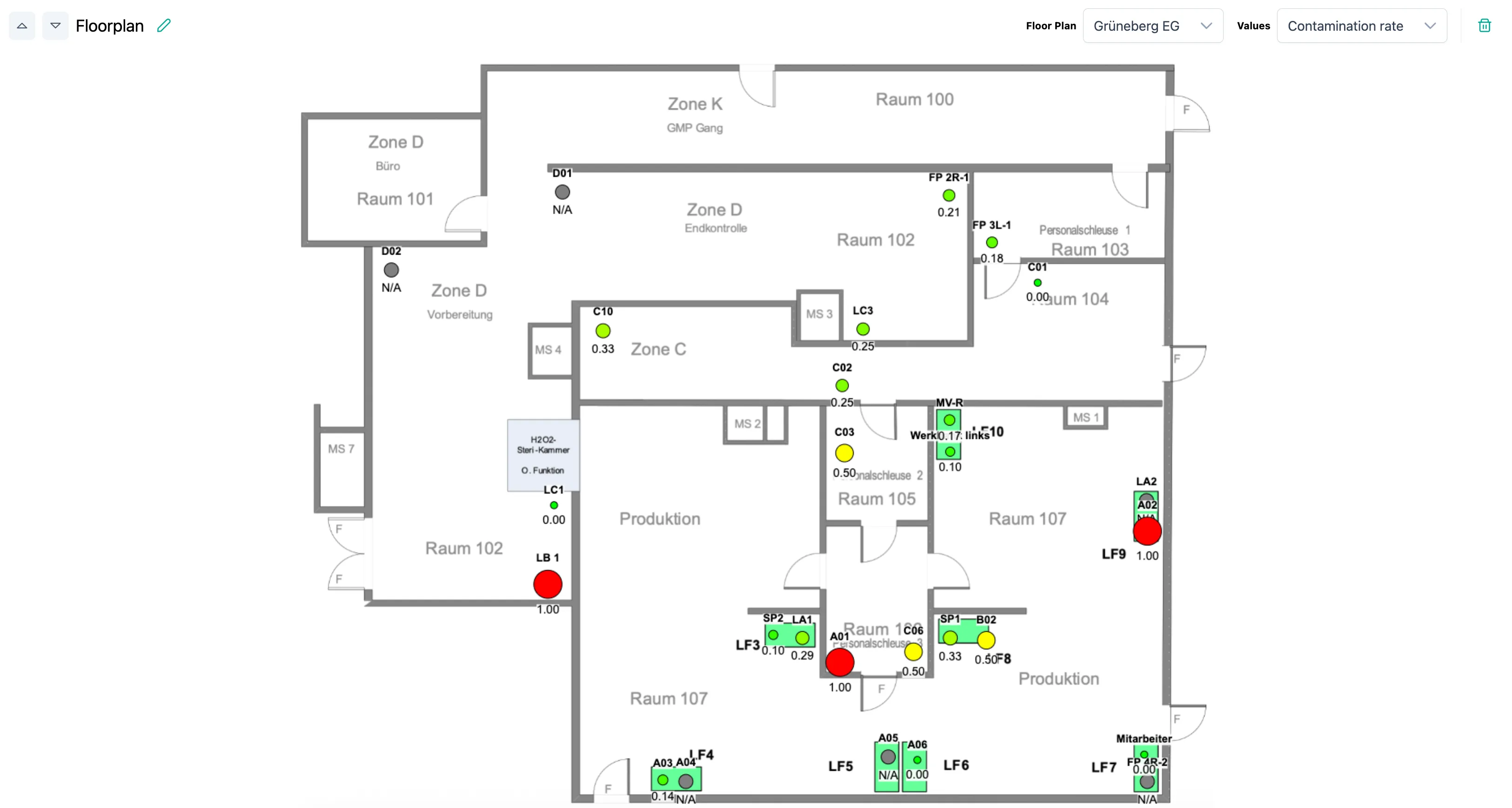
Now new: Floor plans
See at a glance where action is needed – directly on your building plan. Simply upload the floor plan, place measurement points, and identify trends.
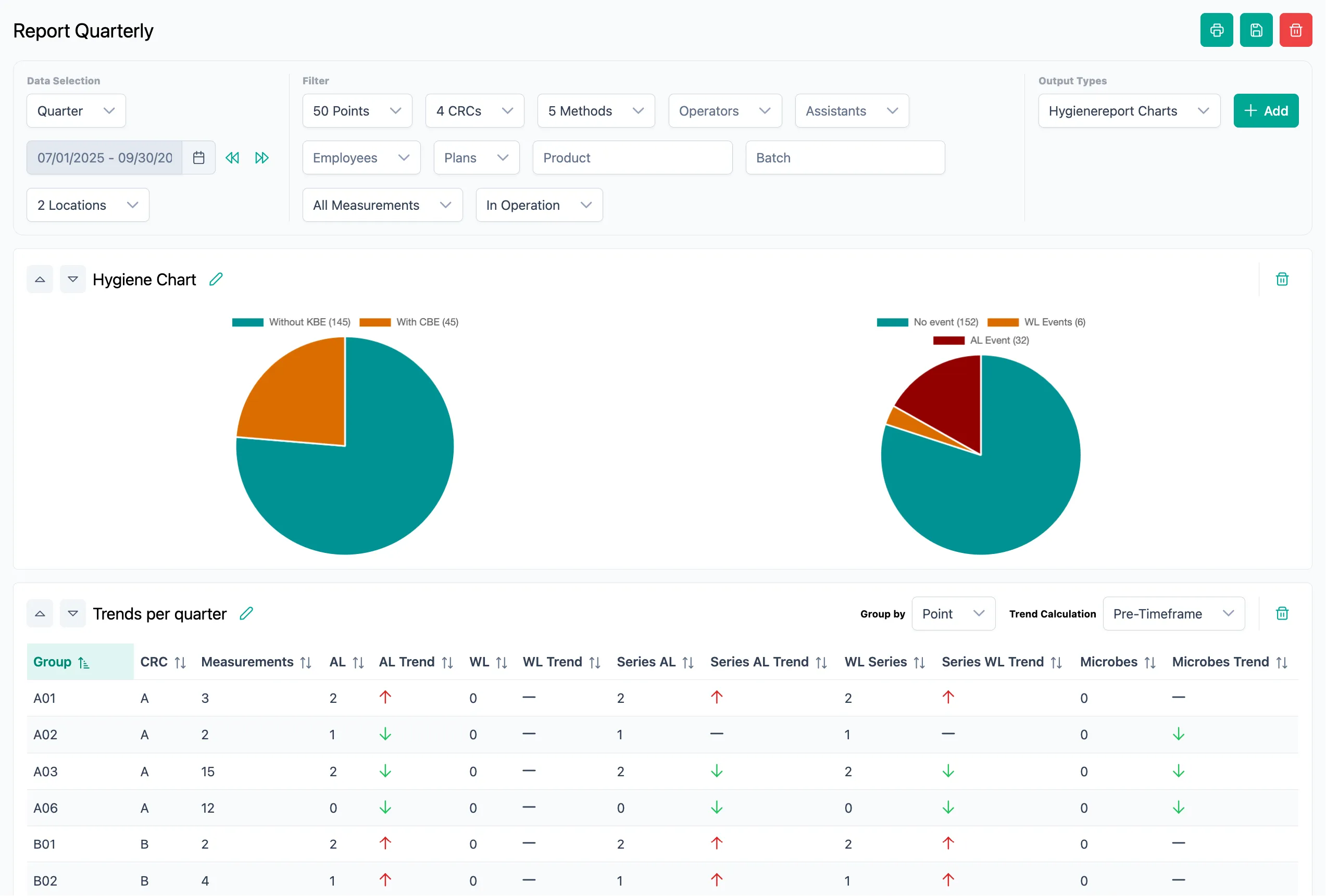
Microbiological environmental monitoring easier than ever
All processes clearly bundled in one application
Deviations
Trend Analysis
Validations
Barcode Support
Schedules & Reminders
Validated Software
Customized Sample Plans
With SteriTrend, you always have all processes in view. Clear daily or weekly views allow you to easily plan and process your microbiological monitoring. Of course, approvals and evaluations are also part of the application.
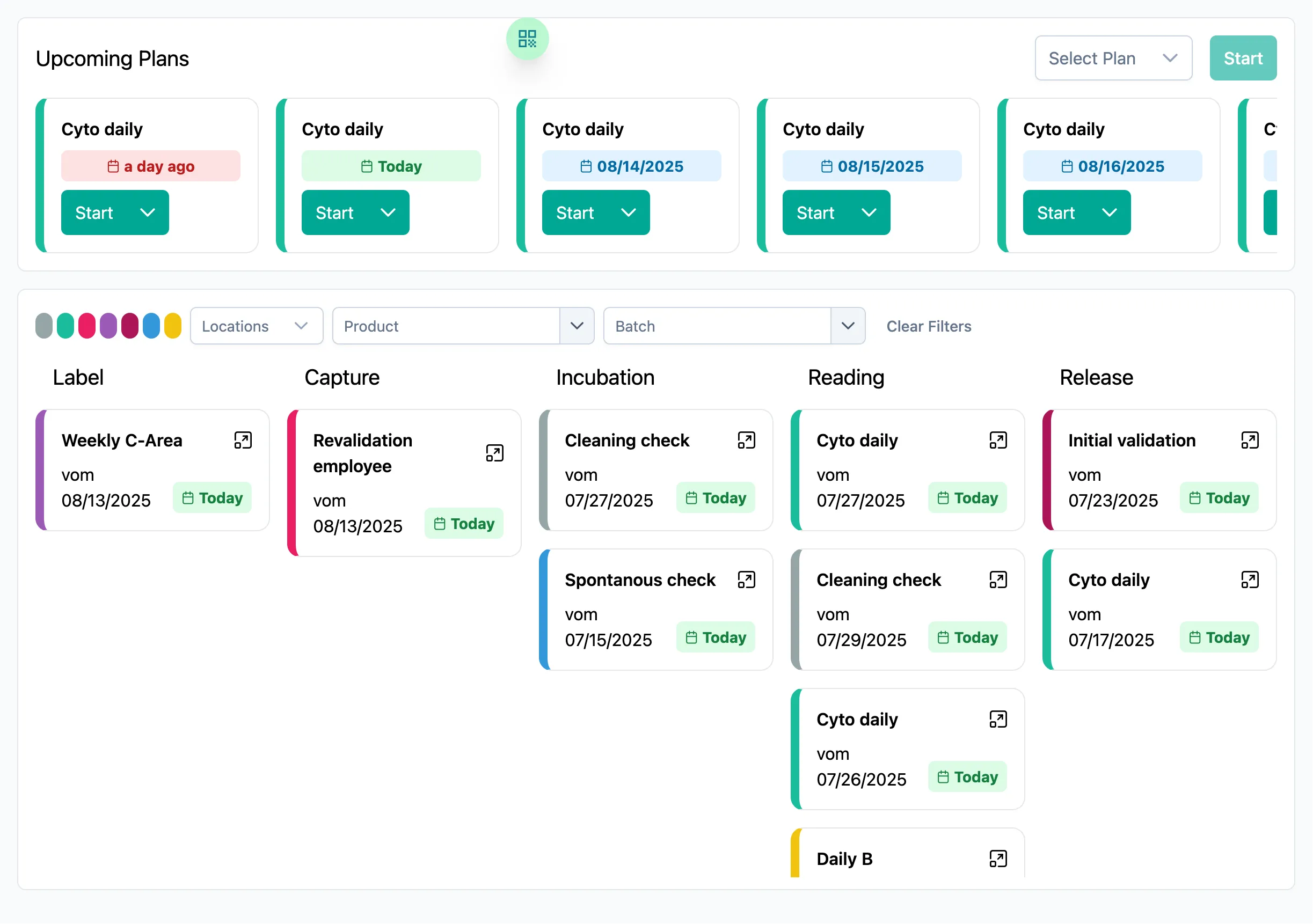
Easy Guidance
You are guided step by step through upcoming measurements, avoiding errors.
Goodbye Excel Chaos
GMP-compliant documentation of all processes is created in SteriTrend, so you are prepared for the next inspection.
Clear Evaluation
You can see your microbiological status directly and react immediately.
Book Your Demo Now
In a 60-minute demo, we'll show you the details of our trends, analyses and evaluations for your individual use case.