Validate employees and processes
Capture and evaluate validations automatically
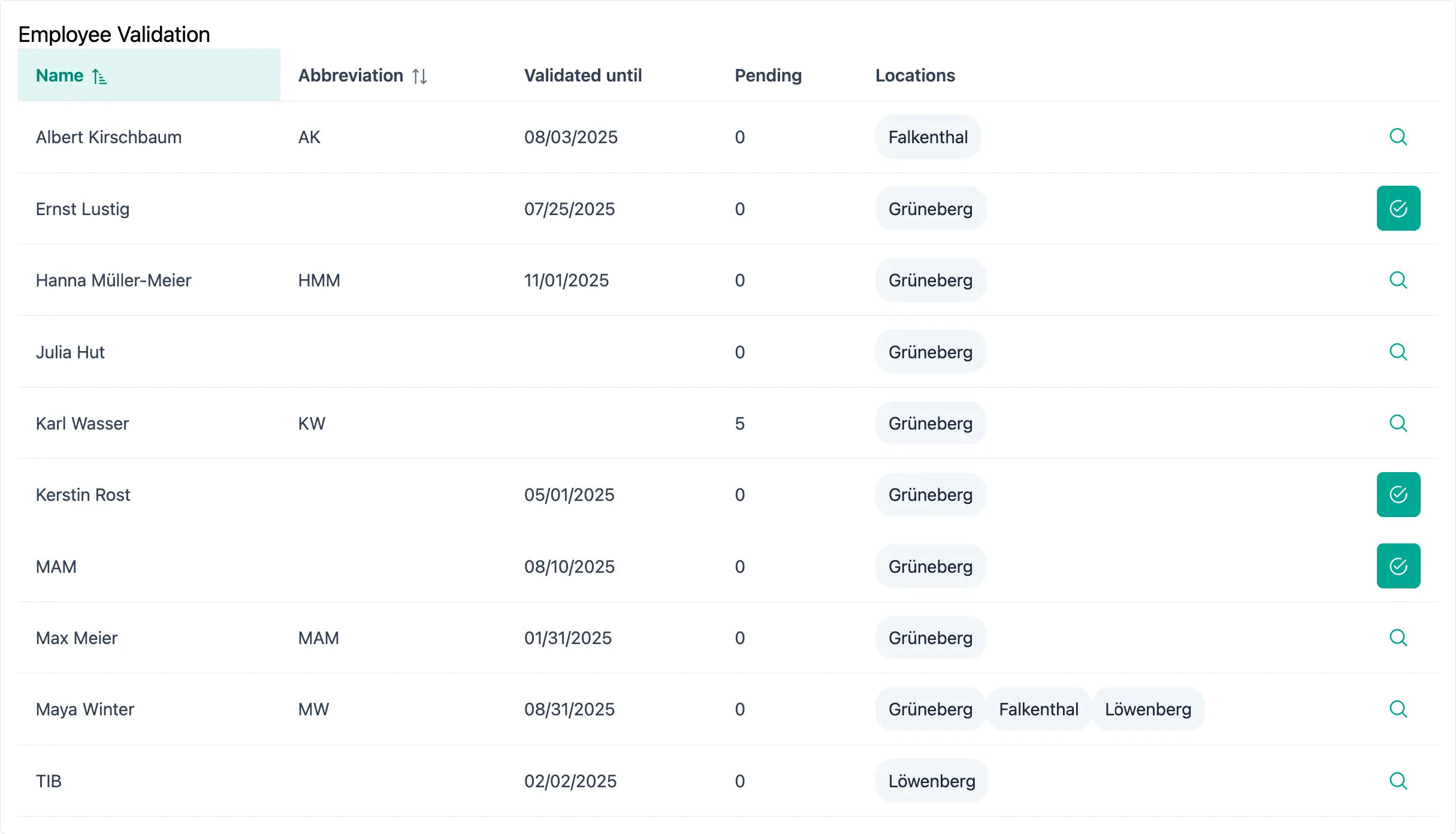
Employee Validation
Automated
Let the system automatically record initial and revalidation. As soon as the required number of successful manufacturing simulations has been recorded, the employee's status is automatically adjusted.
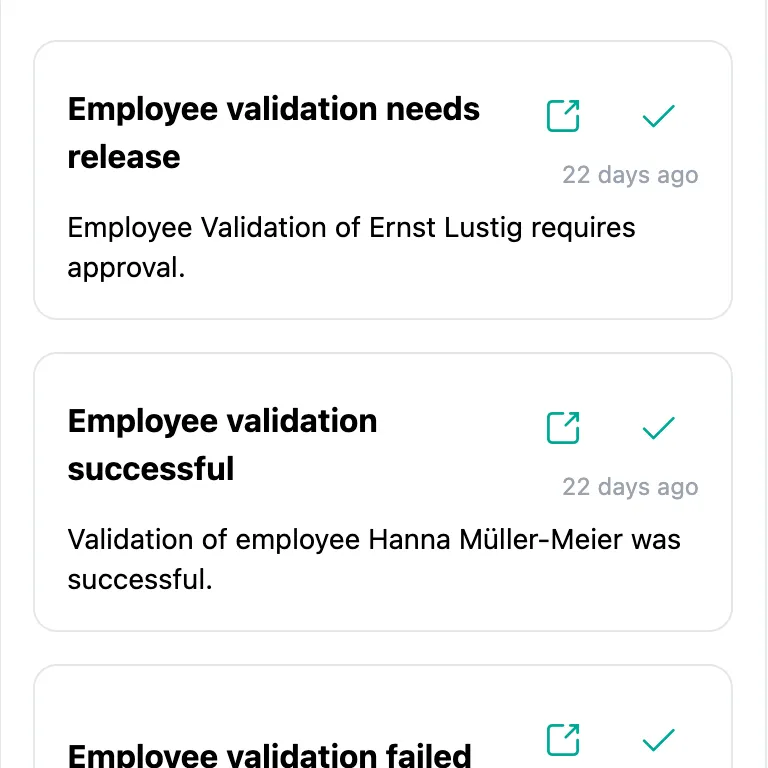
Notifications
Reminders and notifications about relevant events, such as upcoming validation expiries, successful revalidation, or failed attempts. Both via email and as messages in SteriTrend. Individually configurable for each user.
Process Validation
Relevant processes continuously monitored and their safety documented
Documentation
Seamlessly document and evaluate filter changes, cleanroom modifications, and other events through cleaning controls to always maintain a complete overview.
In-Use Validation
Multiple withdrawals from the original container? Interim storage in the refrigerator? In-use validation in SteriTrend reliably supports you and guarantees GMP-compliant work.
Positive Controls
Do you need your own growth evidence in addition to the test certificates from media manufacturers, e.g., with your facility-specific environmental isolates? The positive controls in SteriTrend enable you to do this without distorting the statistics.
Book Your Demo Now
In a 60-minute demo, we'll be happy to show you the details of our validation functions for your individual use case.